Bald Eagle Haliaeetus leucocephalus Scientific name definitions
- LC Least Concern
- Names (35)
- Subspecies (2)
David A. Buehler
Version: 2.0 — Published October 7, 2022
Revision Notes
Revision Notes
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Basque | Itsas arrano buruzuria |
| Bulgarian | Белоглав орел |
| Catalan | pigarg americà |
| Croatian | bjeloglavi štekavac |
| Czech | orel bělohlavý |
| Danish | Hvidhovedet Havørn |
| Dutch | Amerikaanse Zeearend |
| English | Bald Eagle |
| English (United States) | Bald Eagle |
| Finnish | valkopäämerikotka |
| French | Pygargue à tête blanche |
| French (France) | Pygargue à tête blanche |
| German | Weißkopf-Seeadler |
| Greek | Λευκοκέφαλος Θαλασσαετός |
| Hebrew | עיטם לבן-ראש |
| Hungarian | Fehérfejű rétisas |
| Icelandic | Skallaörn |
| Japanese | ハクトウワシ |
| Lithuanian | Baltagalvis jūrinis erelis |
| Norwegian | hvithodehavørn |
| Polish | bielik amerykański |
| Portuguese (Portugal) | Pigargo-americano |
| Romanian | Codalb cu cap alb |
| Russian | Белоголовый орлан |
| Serbian | Beloglavi belorepan (beloglavi orao) |
| Slovak | orliak bielohlavý |
| Slovenian | Ameriški jezerec |
| Spanish | Pigargo Americano |
| Spanish (Cuba) | Aguila calva |
| Spanish (Mexico) | Águila Cabeza Blanca |
| Spanish (Puerto Rico) | Águila Calva |
| Spanish (Spain) | Pigargo americano |
| Swedish | vithövdad havsörn |
| Turkish | Ak Başlı Kartal |
| Ukrainian | Орлан білоголовий |
Revision Notes
David A. Buehler revised the text, with contributions by Peter Pyle on the "Plumages, Molts, and Structure" page, Guy M. Kirwan on the "Systematics" page, and Andrew J. Spencer on the "Sounds and Vocal Behaviors" page. Steven G. Mlodinow edited and copy edited the account. Claire Walter also copy edited the account. Rachel E. Post and Qwahn Kent managed the references. August Davidson-Onsgard and Arnau Bonan Barfull curated the media. Ricardo Cruz updated the distribution map.
Haliaeetus leucocephalus (Linnaeus, 1766)
PROTONYM:
Falco leucocephalus
Linnaeus, 1766. Systema Naturæ. Editio duodecima reformata. Tomus I [part 1], p.124.
TYPE LOCALITY:
Carolina, ex Catesby.
SOURCE:
Avibase, 2023
Definitions
- HALIAEETUS
- leucocephala / leucocephalos / leucocephalus
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
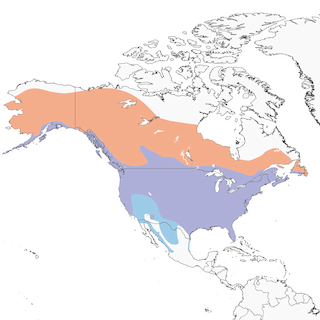
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Bald Eagle