Common Murre Uria aalge Scientific name definitions
- LC Least Concern
- Names (42)
- Subspecies (5)
David G. Ainley, David N. Nettleship, and Anne E. Storey
Version: 2.0 — Published August 6, 2021
Revision Notes
Revision Notes
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Asturian | Arñu comñn |
| Basque | Martin arrunta |
| Bulgarian | Тънкоклюна кайра |
| Catalan | somorgollaire comú |
| Chinese | 崖海鴉 |
| Chinese (SIM) | 崖海鸦 |
| Croatian | tankokljuna njorka |
| Czech | alkoun úzkozobý |
| Danish | Lomvie |
| Dutch | Zeekoet |
| English | Common Murre |
| English (UK) | Common Guillemot |
| English (United States) | Common Murre |
| Faroese | Lomvigi |
| Finnish | etelänkiisla |
| French | Guillemot marmette |
| French (France) | Guillemot marmette |
| Galician | Arao dos cons |
| German | Trottellumme |
| Greek | Λεπτόραμφος Κέπφος |
| Hebrew | אורייה מצויה |
| Hungarian | Lumma |
| Icelandic | Langvía |
| Italian | Uria |
| Japanese | ウミガラス |
| Korean | 바다오리 |
| Latvian | Tievknābja kaira |
| Lithuanian | Laibasnapis narūnėlis |
| Norwegian | lomvi |
| Polish | nurzyk |
| Portuguese (Portugal) | Airo |
| Romanian | Alcă nordică |
| Russian | Тонкоклювая кайра |
| Serbian | Tankokljuna njorka |
| Slovak | norec tenkozobý |
| Slovenian | Lumna |
| Spanish | Arao Común |
| Spanish (Mexico) | Arao Común |
| Spanish (Spain) | Arao común |
| Swedish | sillgrissla |
| Turkish | Alk |
| Ukrainian | Кайра тонкодзьоба |
Revision Notes
In this revision, David G. Ainley, David N. Nettleship, and Anne E. Storey revised all content. Peter Pyle contributed to the Appearance page. Arnau Bonan Barfull, Brooke Keeney, and Peter Pyle curated the media.
Uria aalge (Pontoppidan, 1763)
PROTONYM:
Colymbus aalge
Pontoppidan, 1763. Den Danske Atlas eller Konge-Riget Dannemark (etc.) 1, p.621 pl.26.
TYPE LOCALITY:
Iceland.
SOURCE:
Avibase, 2023
Definitions
- URIA
- aalge
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
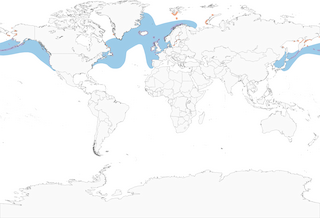
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Common Murre





























