Great Blue Heron Ardea herodias Scientific name definitions
- Names (43)
- Subspecies (5)
Ross G. Vennesland and Robert W. Butler
Version: 1.0 — Published March 4, 2020
Text last updated April 28, 2011
Text last updated April 28, 2011
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Afrikaans | Grootbloureier |
| Bulgarian | Голяма синя чапла |
| Catalan | bernat americà |
| Croatian | američka čaplja |
| Czech | volavka velká |
| Danish | Stor Blåhejre |
| Dutch | Amerikaanse Blauwe Reiger |
| English | Great Blue Heron |
| English (United States) | Great Blue Heron |
| Finnish | amerikanharmaahaikara |
| French | Grand Héron |
| French (France) | Grand Héron |
| German | Kanadareiher |
| Greek | Αμερικανικός Σταχτοτσικνιάς |
| Haitian Creole (Haiti) | Gwo Krabye ble |
| Hebrew | אנפה אמריקנית |
| Hungarian | Királygém |
| Icelandic | Bláhegri |
| Japanese | オオアオサギ |
| Lithuanian | Didysis pilkasis garnys |
| Norwegian | herodiashegre |
| Polish | czapla modra |
| Portuguese (Brazil) | garça-azul-grande |
| Portuguese (Portugal) | Garça-real-americana |
| Romanian | Stârc cu umeri negri |
| Russian | Большая голубая цапля |
| Serbian | Velika plava čaplja |
| Slovak | volavka statná |
| Slovenian | Ameriška siva čaplja |
| Spanish | Garza Azulada |
| Spanish (Costa Rica) | Garzón Azulado |
| Spanish (Cuba) | Garcilote azul |
| Spanish (Dominican Republic) | Garzón Cenizo |
| Spanish (Ecuador) | Garzón Azul |
| Spanish (Honduras) | Garzón Azul |
| Spanish (Mexico) | Garza Morena |
| Spanish (Panama) | Garza Azul Mayor |
| Spanish (Puerto Rico) | Garzón Cenizo |
| Spanish (Spain) | Garza azulada |
| Spanish (Venezuela) | Garzón Cenizo |
| Swedish | amerikansk gråhäger |
| Turkish | Amerika Gri Balıkçılı |
| Ukrainian | Чапля північна |
Ardea herodias Linnaeus, 1758
PROTONYM:
Ardea Herodias
Linnaeus, 1758. Systema Naturæ per Regna Tria Naturæ, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Tomus I. Editio decima, reformata 1, p.143.
TYPE LOCALITY:
America = Hudson Bay, ex Edwards.
SOURCE:
Avibase, 2023
Definitions
- ARDEA
- herodias
- Herodias
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
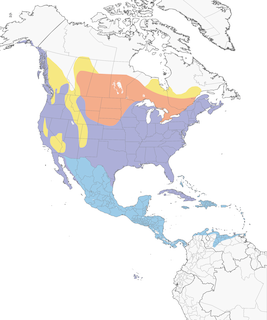
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Great Blue Heron



























