Isabelline Wheatear Oenanthe isabellina Scientific name definitions
- LC Least Concern
- Names (47)
- Monotypic
Nigel Collar
Version: 1.0 — Published March 4, 2020
Text last updated December 21, 2013
Text last updated December 21, 2013
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Afrikaans | Isabellaskaapwagter |
| Albanian | Bishtëbardha vetullbardhë |
| Arabic | أبلق أشهب |
| Armenian | Պարող քարաթռչնակ |
| Asturian | Peñiquina isabelina |
| Azerbaijani | Oynaq çaxraqçıl |
| Basque | Buztanzuri isabeldarra |
| Bulgarian | Ориенталско каменарче |
| Catalan | còlit isabelí |
| Chinese | 沙䳭 |
| Chinese (SIM) | 沙䳭 |
| Croatian | azijska bjeloguza |
| Czech | bělořit plavý |
| Danish | Isabellastenpikker |
| Dutch | Izabeltapuit |
| English | Isabelline Wheatear |
| English (United States) | Isabelline Wheatear |
| Finnish | arotasku |
| French | Traquet isabelle |
| French (France) | Traquet isabelle |
| Galician | Pedreiro isabel |
| German | Isabellsteinschmätzer |
| Greek | Αμμοπετρόκλης |
| Hebrew | סלעית ערבות |
| Hungarian | Pusztai hantmadár |
| Icelandic | Steppudepill |
| Italian | Culbianco isabellino |
| Japanese | イナバヒタキ |
| Korean | 긴다리사막딱새 |
| Lithuanian | Palšasis kūltupys |
| Malayalam | നെന്മണിക്കുരുവി |
| Mongolian | Бүжимч чогчиго |
| Norwegian | isabellasteinskvett |
| Persian | چکچک دشتی |
| Polish | białorzytka płowa |
| Portuguese (Portugal) | Chasco-isabel |
| Romanian | Pietrar răsăritean |
| Russian | Каменка-плясунья |
| Serbian | Stepska beloguza |
| Slovak | skaliarik plavý |
| Slovenian | Bledi kupčar |
| Spanish | Collalba Isabel |
| Spanish (Spain) | Collalba isabel |
| Swedish | isabellastenskvätta |
| Thai | นกเขนทะเลทราย |
| Turkish | Boz Kuyrukkakan |
| Ukrainian | Кам’янка попеляста |
Oenanthe isabellina (Temminck, 1829)
PROTONYM:
Saxicola isabellina
Temminck, 1829. Nouveau recueil de planches coloriées d'oiseaux, pour servir de suite et de complément aux planches enluminées de Buffon livr.79, pl.472 fig.1.
TYPE LOCALITY:
Nubia.
SOURCE:
Avibase, 2023
Definitions
- OENANTHE
- oenanthe
- isabellina / isabellinus
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
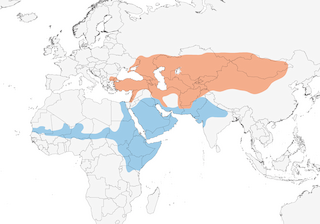
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Isabelline Wheatear




























