Northern Wheatear Oenanthe oenanthe Scientific name definitions
- LC Least Concern
- Names (53)
- Subspecies (3)
Erica H. Dunn, David J. T. Hussell, Josef Kren, and Amelia C. Zoerb
Version: 2.1 — Published October 25, 2022
Revision Notes
Version: 2.1 — Published October 25, 2022
Revision Notes
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Afrikaans | Europese Skaapwagter |
| Albanian | Bishtbardha e gurit |
| Arabic | أبلق شمالي |
| Armenian | Սովորական քարաթռչնակ |
| Asturian | Peñiquina buxa |
| Azerbaijani | Adi çaxraqçıl |
| Basque | Buztanzuri arrunta |
| Bulgarian | Сиво каменарче |
| Catalan | còlit gris |
| Chinese | 穗䳭 |
| Chinese (SIM) | 穗䳭 |
| Croatian | sivkasta bjeloguza |
| Czech | bělořit šedý |
| Danish | Stenpikker |
| Dutch | Tapuit |
| English | Northern Wheatear |
| English (United States) | Northern Wheatear |
| Faroese | Steinstólpa |
| Finnish | kivitasku |
| French | Traquet motteux |
| French (France) | Traquet motteux |
| Galician | Pedreiro cincento |
| German | Steinschmätzer |
| Greek | Σταχτοπετρόκλης |
| Hebrew | סלעית אירופית |
| Hungarian | Hantmadár |
| Icelandic | Steindepill |
| Italian | Culbianco |
| Japanese | ハシグロヒタキ |
| Korean | 북방사막딱새 |
| Latvian | Akmeņčakstīte |
| Lithuanian | Paprastasis kūltupys |
| Malayalam | വടക്കൻ നെന്മണിക്കുരുവി |
| Mongolian | Адууч чогчиго |
| Norwegian | steinskvett |
| Persian | چکچک کوهی |
| Polish | białorzytka |
| Portuguese (Portugal) | Chasco-cinzento |
| Romanian | Pietrar sur |
| Russian | Обыкновенная каменка |
| Serbian | Obična beloguza |
| Slovak | skaliarik sivý |
| Slovenian | Navadni kupčar |
| Spanish | Collalba Gris |
| Spanish (Cuba) | Tordo ártico |
| Spanish (Mexico) | Collalba Norteña |
| Spanish (Panama) | Collalba Gris |
| Spanish (Puerto Rico) | Collalba Gris |
| Spanish (Spain) | Collalba gris |
| Swedish | stenskvätta |
| Thai | นกเขนทะเลทรายแถบเหนือ |
| Turkish | Kuyrukkakan |
| Ukrainian | Кам’янка звичайна |
Revision Notes
Paul G. Rodewald standardized the content with Clements taxonomy. Peter Pyle contributed to the Plumages, Molts, and Structure page. Shawn M. Billerman contributed to the Systematics page. Claire Walter copyedited the references.
Oenanthe oenanthe (Linnaeus, 1758)
PROTONYM:
Motacilla oenanthe
Linnaeus, 1758. Systema Naturæ per Regna Tria Naturæ, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Tomus I. Editio decima, reformata 1, p.186.
TYPE LOCALITY:
in Europae apricis lapidosis [= Sweden, fide Hartert, 1910, Vog. pal. Fauna, p. 679].
SOURCE:
Avibase, 2023
Definitions
- OENANTHE
- oenanthe
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
Account navigation Account navigation
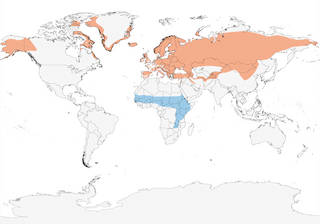
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Northern Wheatear