Purple Martin Progne subis Scientific name definitions
- LC Least Concern
- Names (42)
- Subspecies (3)
Charles R. Brown, Daniel A. Airola, and Scott Tarof
Version: 2.0 — Published September 10, 2021
Revision Notes
Revision Notes
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | oreneta porpra |
| Croatian | veliki piljak |
| Czech | jiřička modrolesklá |
| Danish | Purpursvale |
| Dutch | Purperzwaluw |
| English | Purple Martin |
| English (United States) | Purple Martin |
| French | Hirondelle noire |
| French (France) | Hirondelle noire |
| German | Purpurschwalbe |
| Greek | Πορφυροχελίδονο |
| Haitian Creole (Haiti) | Irondèl vyolèt |
| Hungarian | Biborfescke |
| Icelandic | Purpurasvala |
| Japanese | ムラサキツバメ |
| Lithuanian | Purpurinė miškinė kregždė |
| Norwegian | purpursvale |
| Polish | jaskółczak modry |
| Portuguese (Brazil) | andorinha-azul |
| Portuguese (Portugal) | Andorinha-azul |
| Romanian | Lăstun purpuriu |
| Russian | Большая ласточка |
| Serbian | Ljubičasta lasta |
| Slovak | lastovička purpurová |
| Spanish | Golondrina Purpúrea |
| Spanish (Argentina) | Golondrina Purpúrea |
| Spanish (Costa Rica) | Martín Purpúrea |
| Spanish (Cuba) | Golondrina azul americana |
| Spanish (Dominican Republic) | Golondrina Migratoria |
| Spanish (Ecuador) | Martín Purpúreo |
| Spanish (Honduras) | Golondrina Púrpura |
| Spanish (Mexico) | Golondrina Azulnegra |
| Spanish (Panama) | Martín Purpúreo |
| Spanish (Paraguay) | Golondrina purpúrea |
| Spanish (Peru) | Martín Purpúreo |
| Spanish (Puerto Rico) | Golondrina Púrpura |
| Spanish (Spain) | Golondrina purpúrea |
| Spanish (Uruguay) | Golondrina Purpúrea |
| Spanish (Venezuela) | Golondrina de Iglesias |
| Swedish | blå storsvala |
| Turkish | Büyük Mor Kırlangıç |
| Ukrainian | Щурик пурпуровий |
Revision Notes
In this revision, Charles R. Brown and Daniel A. Airola revised all content. Peter Pyle contributed to the Appearance page. Arnau Bonan Barfull and Peter Pyle curated the media.
Progne subis (Linnaeus, 1758)
PROTONYM:
Hirundo subis
Linnaeus, 1758. Systema Naturæ per Regna Tria Naturæ, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Tomus I. Editio decima, reformata 1, p.192.
TYPE LOCALITY:
Hudson Bay.
SOURCE:
Avibase, 2023
Definitions
- PROGNE
- progne
- subis
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
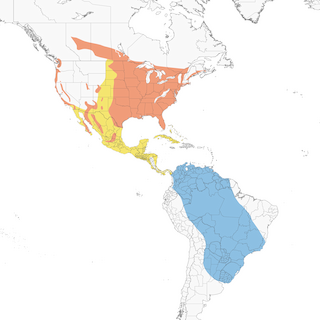
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Purple Martin































