Red-throated Loon Gavia stellata Scientific name definitions
- LC Least Concern
- Names (48)
- Monotypic
Daniel J. Rizzolo, Carrie E. Gray, Joel A. Schmutz, Jack F. Barr, Christine Eberl, and Judith W. McIntyre
Version: 2.0 — Published April 16, 2020
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Albanian | Nori gushëkuq |
| Arabic | غواص أحمر الحنجرة |
| Armenian | Կարմրախածի սուզահավ |
| Asturian | Mobeya pequeña |
| Azerbaijani | Qırmızıdöş qaqar |
| Basque | Aliota txikia |
| Bulgarian | Червеногуш гмуркач |
| Catalan | calàbria petita |
| Chinese | 紅喉潛鳥 |
| Chinese (SIM) | 红喉潜鸟 |
| Croatian | crvenogrli plijenor |
| Czech | potáplice malá |
| Danish | Rødstrubet Lom |
| Dutch | Roodkeelduiker |
| English | Red-throated Loon |
| English (UK) | Red-throated Diver |
| English (United States) | Red-throated Loon |
| Faroese | Øsreyður lómur |
| Finnish | kaakkuri |
| French | Plongeon catmarin |
| French (France) | Plongeon catmarin |
| Galician | Mobella pequena |
| German | Sterntaucher |
| Greek | Κηλιδοβούτι |
| Hebrew | צוללן אדום-גרון |
| Hungarian | Északi búvár |
| Icelandic | Lómur |
| Italian | Strolaga minore |
| Japanese | アビ |
| Korean | 아비 |
| Latvian | Brūnkakla gārgale |
| Lithuanian | Rudakaklis naras |
| Mongolian | Улаан гүеэт гахууна |
| Norwegian | smålom |
| Persian | غواص گلوسرخ |
| Polish | nur rdzawoszyi |
| Portuguese (Portugal) | Mobelha-pequena |
| Romanian | Cufundar mic |
| Russian | Краснозобая гагара |
| Serbian | Riđogrli morski gnjurac |
| Slovak | potáplica malá |
| Slovenian | Rdečegrli slapnik |
| Spanish | Colimbo Chico |
| Spanish (Mexico) | Colimbo Menor |
| Spanish (Spain) | Colimbo chico |
| Swedish | smålom |
| Turkish | Kızıl Gerdanlı Dalgıç |
| Ukrainian | Гагара червоношия |
Gavia stellata (Pontoppidan, 1763)
PROTONYM:
Colymbus Stellatus
Pontoppidan, 1763. Den Danske Atlas eller Konge-Riget Dannemark (etc.) 1, p.621.
TYPE LOCALITY:
Tame River, Warwickshire, England, ex Willughby (cf. Laubmann, 1922, Verb. Ornith. Gesell. Bayern, 15, p. 211).
SOURCE:
Avibase, 2023
Definitions
- GAVIA
- gavia
- stellata / stellatus
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
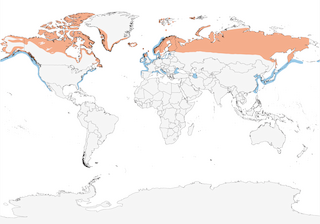
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Red-throated Loon






























