Seaside Sparrow Ammospiza maritima Scientific name definitions
- LC Least Concern
- Names (20)
- Subspecies (7)
Jon S. Greenlaw, W. Gregory Shriver, and William Post
Version: 2.0 — Published July 1, 2022
Revision Notes
Revision Notes
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | sit pardalenc costaner |
| Dutch | Kweldergors |
| English | Seaside Sparrow |
| English (United States) | Seaside Sparrow |
| French | Bruant maritime |
| French (France) | Bruant maritime |
| German | Strandammer |
| Icelandic | Flæðatittlingur |
| Japanese | ハマヒメドリ |
| Norwegian | marskspurv |
| Polish | bagiennik żółtoczelny |
| Russian | Приморская овсянка-барсучок |
| Serbian | Morski strnad |
| Slovak | strnádlik prímorský |
| Spanish | Chingolo Costero |
| Spanish (Mexico) | Gorrión Costero |
| Spanish (Spain) | Chingolo costero |
| Swedish | kustsparv |
| Turkish | Sahil Serçesi |
| Ukrainian | Багновець приморський |
Revision Notes
Jon Greenlaw and Greg Shriver revised the account. Claire Walter managed the references. Guy Kirwan contributed some of the Systematics content. Arnau Bonan Barfull curated the media.
Ammospiza maritima (Wilson, 1811)
PROTONYM:
Fringilla maritima
Wilson, 1811. American Ornithology or, the Natural History of the Birds of the United States: Illustrated with Plates Engraved and Colored from Original Drawings taken from Nature 4, p.68 pl.34 fig.2.
TYPE LOCALITY:
sea islands along our Atlantic coast ; restricted to Great Egg Harbor, New Jersey, by Oberholser, 1931, Proc. Biol. Soc. Washington, 44, p. 124.
SOURCE:
Avibase, 2023
Definitions
- AMMOSPIZA
- maritima / maritimus
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
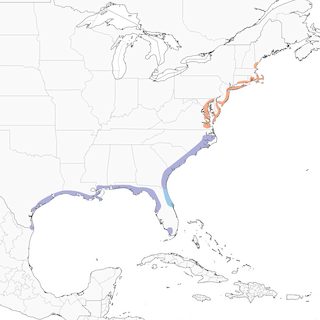
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Seaside Sparrow





























